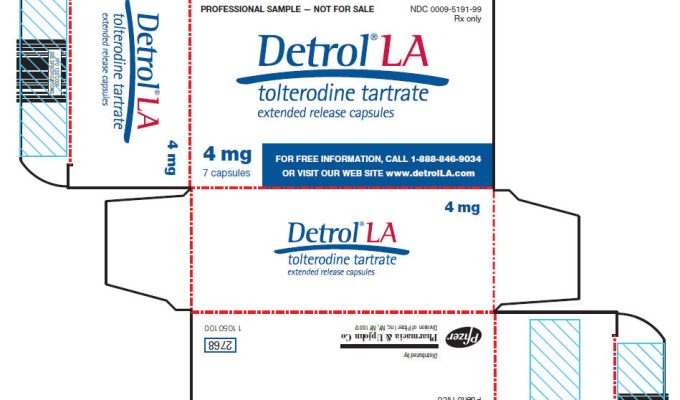
Chennai patent office revokes Pfizer’s patent on Detrol LA
In an interesting development the Chennai Patent Office recently revoked Pfizer’s patent on Detrol LA in response to a post grant opposition initiated by Ranbaxy. Detrol LA (extended release capsules of tolterodine) is used for symptomatic treatment of urinary incontinence/overactive bladder syndrome. The revocation order can be accessed here. Patent status of Detrol LA in India In India Detrol LA is protected by two patents IN211539 and IN229260 which cover its extended release capsule formulation. It is pertinent to note that there […]
Chennai patent office revokes Pfizer’s patent on Detrol LA Read More »