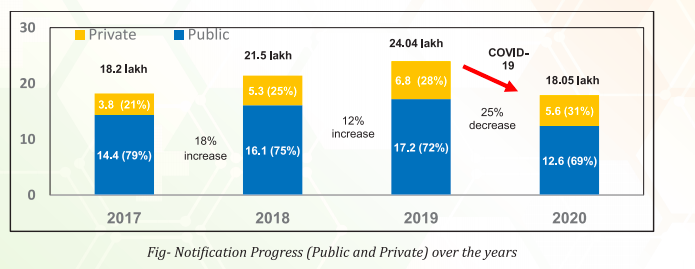
Division Bench Stays the Interim Injunction Granted to Kibow Biotech, calls Methodology of the Single Judge Bench for Establishing Prima Facie case “Flawed”
The present post discusses another instance of interim injunctions imposed by our courts on the basis of problematic rationale. Sometime back, I reported on an interim injunction order by a Single Judge Bench of Madras High Court against La Renon Healthcare Pvt. Ltd. and Stanford Labs Pvt. Ltd (the defendants). Without narrating the background in detail again, the order was passed in an ongoing patent infringement dispute (covered here and here for the blog) surrounding Plaintiff no. 2’s (Kibow Biotech) […]